Introduction
Escherichia coli (EPEC) is an important pathogenic strain of E. coli known for causing diarrheal disease, especially in young children. The detection of EPEC DNA through Polymerase Chain Reaction (PCR) is a widely used technique in clinical and research laboratories. A PCR qualitative positive control is essential in ensuring the accuracy and reliability of EPEC diagnostic assays.
PCR-based methods allow for the specific identification of EPEC DNA in biological samples, ensuring the early detection and monitoring of outbreaks. The Centers for Disease Control and Prevention (CDC) provides detailed protocols for PCR-based detection of enteropathogens (CDC).
Importance of PCR Positive Controls
A PCR positive control is a critical component in molecular diagnostics. It helps in verifying assay performance, preventing false negatives, and ensuring reproducibility. Research institutions such as National Center for Biotechnology Information (NCBI) emphasize the role of PCR controls in pathogen detection.
The Food and Drug Administration (FDA) provides guidelines on the validation of molecular diagnostic tools for infectious diseases (FDA). Similarly, the World Health Organization (WHO) discusses global standards for molecular pathogen detection (WHO).
Applications in Research and Clinical Diagnostics
1. Public Health Surveillance
EPEC is a major public health concern. The National Institutes of Health (NIH) funds research into molecular epidemiology for tracking infectious diseases (NIH).
2. Molecular Epidemiology Studies
Several academic institutions such as Harvard Medical School and Johns Hopkins University are conducting research on EPEC transmission dynamics and its role in global health.
3. Diagnostic Laboratory Testing
The College of American Pathologists (CAP) provides guidelines for clinical PCR testing and ensures that laboratory assays meet the highest standards (CAP).
4. Development of Vaccines and Therapeutics
Research funded by the National Institute of Allergy and Infectious Diseases (NIAID) explores EPEC pathogenesis and potential therapeutic interventions (NIAID).
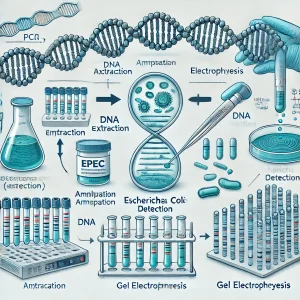
PCR Detection Methods for EPEC DNA
The standard PCR protocol for detecting EPEC DNA involves:
- DNA Extraction from suspected samples (CDC Guidelines).
- Amplification using specific EPEC gene primers, such as eaeA gene, recommended by PubMed.
- Real-time PCR validation to confirm amplification specificity, as discussed in research from Stanford University.
Quality Control and Standardization
To ensure the reliability of PCR results, laboratories must adhere to strict quality control (QC) guidelines. The National Institute of Standards and Technology (NIST) provides protocols for molecular assay validation (NIST).
Furthermore, the European Centre for Disease Prevention and Control (ECDC) shares insights on standardized molecular diagnostics (ECDC).
Challenges and Future Directions
Despite advancements in molecular diagnostics, challenges remain, including:
- PCR inhibitors affecting assay sensitivity.
- Genetic variability of EPEC strains.
- Cost and accessibility of molecular diagnostics in resource-limited settings.
The Bill & Melinda Gates Foundation is funding research to improve affordable molecular diagnostics for developing countries (Gates Foundation).
Conclusion
The EPEC DNA – PCR Qualitative Positive Control is a vital tool in clinical diagnostics and research. It ensures the accuracy, reproducibility, and reliability of molecular tests for detecting EPEC infections. Researchers and health agencies, including CDC, NIH, WHO, and FDA, are continuously working towards improving the sensitivity and specificity of EPEC PCR-based diagnostics.
For further reading and latest research updates, explore resources at:
The rapid development of molecular diagnostic tools will continue to play a pivotal role in infectious disease research and public health surveillance.