The Annexin A2 ELISA is widely used in biological and biochemical research to detect and measure the concentration of Annexin A2 (ANXA2) protein in samples such as serum, plasma, cell culture supernatants, and tissue lysates. This protein is involved in several biological systems, including membrane transport, cytoskeletal interaction, and extracellular signaling. The ELISA method, built upon immunological specificity and signal amplification, allows precise quantification across a variety of experimental setups.
For foundational information about this protein, visit NCBI Gene – ANXA2, and for protein structure data, refer to RCSB Protein Data Bank.
Structure and Role of Annexin A2
Annexin A2 is a calcium-dependent phospholipid-binding protein with a core role in vesicular trafficking and membrane-cytoskeleton dynamics. It has a conserved C-terminal domain, typical of the annexin family, and a unique N-terminal sequence enabling heterotetramer formation with S100A10.
Functional studies from nih.gov suggest its involvement in membrane repair, endocytic pathways, and surface receptor organization. For detailed insights into annexin family proteins, the PubMed Central database provides open-access literature.
Principles of the ELISA Technique
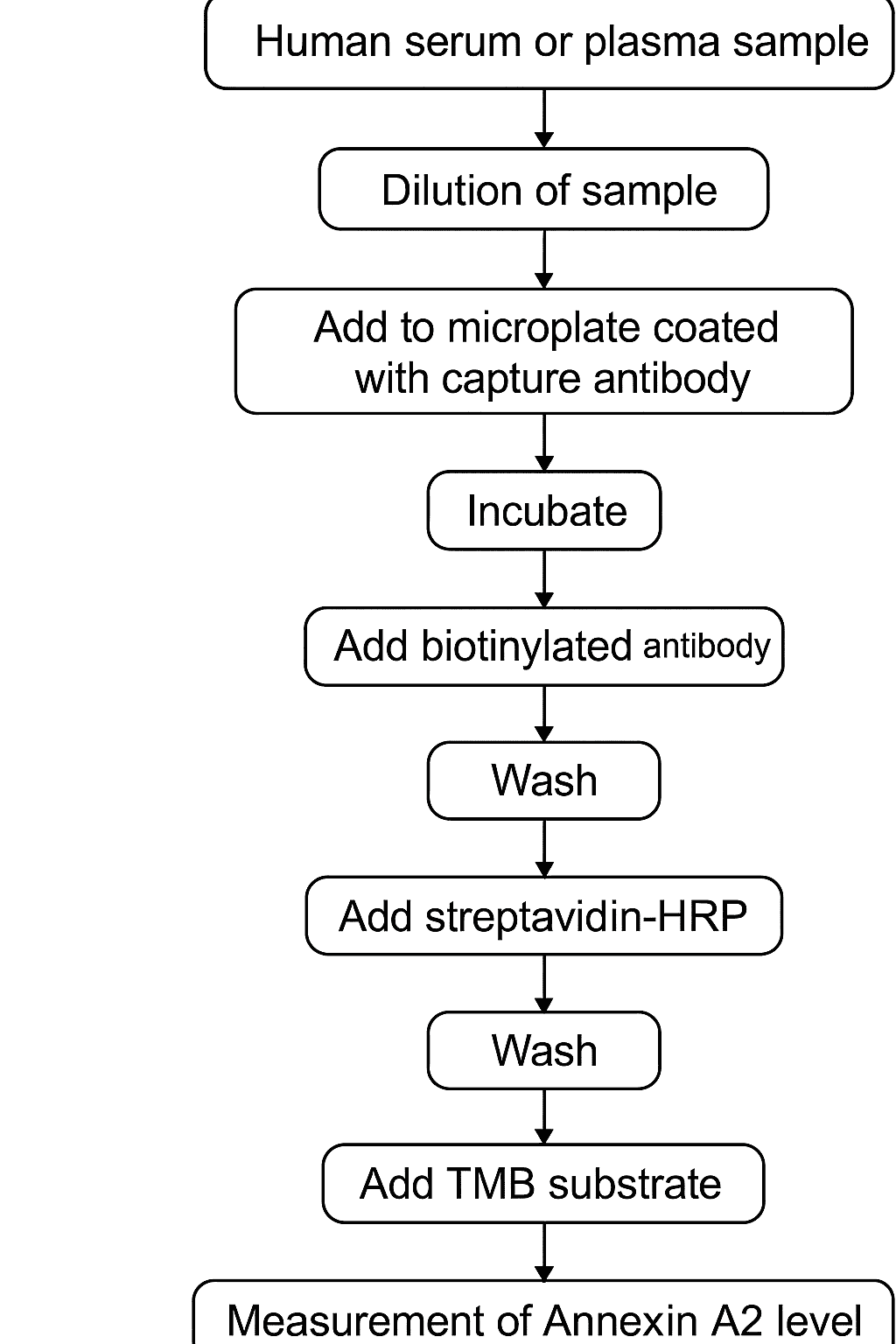
The Annexin A2 ELISA typically follows the sandwich ELISA format, known for its high specificity and sensitivity. This format involves:
-
A 96-well plate coated with capture antibodies against Annexin A2.
-
Addition of the sample or standard protein.
-
A biotinylated detection antibody that binds to a separate epitope.
-
Enzyme-conjugated streptavidin (commonly HRP).
-
A colorimetric substrate like TMB (3,3′,5,5′-Tetramethylbenzidine).
-
Quantification at 450 nm using a plate reader.
Protocols can be adapted from assayguidemanual.ncbi.nlm.nih.gov, which explains assay optimization and performance metrics.
Sample Types and Processing
Annexin A2 can be measured in several biological matrices. Common sample types include:
-
Human and rodent serum/plasma
-
Conditioned medium from cell cultures
-
Tissue homogenates
-
Lysates from adherent or suspension cells
For sample prep guides, see protocols published by labs at jhu.edu, umich.edu, and ucsf.edu.
When preparing tissue lysates, it is essential to maintain cold temperatures and use protease inhibitors, which prevent degradation of target proteins. Buffer selection and homogenization protocols may vary by sample type, as described in ncbi.nlm.nih.gov.
Analytical Range and Sensitivity
Most commercial and academic Annexin A2 ELISA kits detect protein concentrations in the range of 10 pg/mL to 2 ng/mL. High sensitivity can be achieved through:
-
High-affinity monoclonal antibodies
-
Minimal background from nonspecific binding
-
Strict adherence to incubation and wash times
Guidelines for validating analytical parameters such as linearity, LOD (limit of detection), and inter/intra-assay variability are available at fda.gov and in ELISA validation literature indexed in PubMed.
Storage Conditions and Reagent Stability
Annexin A2 ELISA kits contain temperature-sensitive reagents. Recommendations include:
-
Store plates and antibodies at 2–8°C
-
Avoid repeated freeze-thaw cycles for protein standards
-
Use reconstituted detection reagents within 24 hours
Temperature stability data are often reported in supplemental files from labs at cornell.edu, osu.edu, and wisc.edu.
Applications of Annexin A2 Detection
1. Membrane Organization Studies
Annexin A2 interacts with phosphatidylserine in a Ca2+-dependent manner, mediating its recruitment to cell membranes. This property is crucial in:
-
Exocytosis
-
Membrane fusion
-
Endosomal transport
Refer to membrane dynamics studies at cellimagelibrary.org and nih.gov.
2. Interaction With S100A10
In many contexts, Annexin A2 forms a heterotetrameric complex with S100A10, enabling localization at plasma membrane sites. The complex regulates:
-
Ion channel trafficking
-
Actin filament remodeling
-
Exocytic machinery
These interactions are characterized in detail in PMCID: PMC2932715.
3. Cellular Differentiation and Development
Annexin A2 is temporally expressed during embryogenesis, stem cell commitment, and differentiation, as studied in developmental biology labs at stanford.edu and duke.edu.
Protocol Optimization
Blocking Agents
Non-specific binding can be reduced using BSA, casein, or normal serum. Optimal blocking buffers are listed in the Assay Guidance Manual.
Incubation Time
-
Capture antibody coating: Overnight at 4°C
-
Sample binding: 1–2 hours at room temperature
-
Detection: 1 hour
-
Substrate development: 15–30 minutes
Refer to kinetic studies from nih.gov to fine-tune these parameters.
Data Analysis
Standard curves are fitted using:
-
Four-parameter logistic (4-PL) models
-
Linear regression on log-transformed data
Plate readers from academic cores, such as those at uchicago.edu, provide built-in ELISA analysis tools. Exported OD data can be plotted in r-project.org or niddk.nih.gov.
Known Cross-Reactivity
Most ELISA assays for Annexin A2 are highly specific, with negligible cross-reactivity to Annexin A1 or A5. Data on antibody validation are available at antibodyregistry.org and ncbi.nlm.nih.gov.
Safety Considerations
Laboratory practices follow biosafety level 2 (BSL-2) recommendations, especially when handling human-derived samples. Reference safety data from osha.gov and epa.gov.
Limitations and Troubleshooting
-
No signal: Verify activity of detection antibody; avoid expired TMB.
-
High background: Use fresh blocking buffer and increase wash cycles.
-
Curve compression: Dilute high-concentration samples to fall within range.
A full troubleshooting guide is available in the ELISA Troubleshooting Manual – NIH.
Final Remarks
The Annexin A2 ELISA is a powerful method for basic and applied research. By providing sensitive, reproducible measurements of a multifunctional protein, it helps characterize cellular systems across multiple domains. The resources shared from .gov and .edu sources enrich the reproducibility and transparency of your protocols, whether you’re working in membrane biology, intracellular trafficking, or cell differentiation research.
Would you like this exported as Markdown or HTML for integration into your blog system?
![AffiELISA® Pig Annexin A2 ELISA [ ANXA2]](https://affigen.com/cdn/shop/files/5BAFG-E4345_5D_20AffiELISA_C2_AE_20Cattle_20IFNg_20Kit_20High-Resolution_20Interferon_20Gamma_20ELISA_20Detection_61f99d34-fedc-49d5-99cf-2c9035f9faac_535x.png?v=1712832853)